We may think of ourselves as individual organisms, but humans could not survive without a symbiotic relationship with the trillions of microorganisms that inhabit every part of our bodies inside and out. We have evolved to rely on these organisms, and research in recent years has been slowly uncovering their effects on our health. The largest bacterial community, or microbiome, is in our gut. We know they help us digest our food, and they release compounds and nutrients which affect us in various ways. We want to know what we can do to maintain a healthy and balanced microbiome to facilitate good health.
Home testing – what exactly is inhabiting our gut?
Companies like uBioDiscovery (a RIC Centre client) and uBiome are enabling individuals by providing home tests that show us which bacteria are in our gut. To identify what a person’s community of gut bacteria looks like, a fecal sample must be taken to perform genetic sequencing on all the bacteria present. By looking at specific genetic sequences and the relative amounts of each, the species and strains of the organisms as well as relative quantities of each can be determined. Then, these microbiome profiles can be compared between healthy and unhealthy people to see if there’s a pattern.
Some scientists are weary of the limitations of at home testing since there’s a lot more to discover and understand about the relationship between the gut microbiome and disease. Therefore, customers should be cautious and refrain from self-diagnosing disease or making drastic lifestyle changes without consulting a doctor. This is one tool among many in understanding more about our state of health.
For those interested in knowing what their gut bacteria profile looks like, uBioDiscovery offers a kit called Superbiome which provides individuals with a complete analysis of the bacterial community living inside their gut. The kit comes with two sampling tubes and a 30-day supply of probiotics. With two tests, one can get a baseline and then see the effect that diet changes or probiotics has on their microbial community. Customers will also be able to log into an online portal and get a detailed report and chart outlining their results. Most importantly, by generating more data and sharing it with the research community, we will develop better ways to treat disease without the long-term use of drugs!
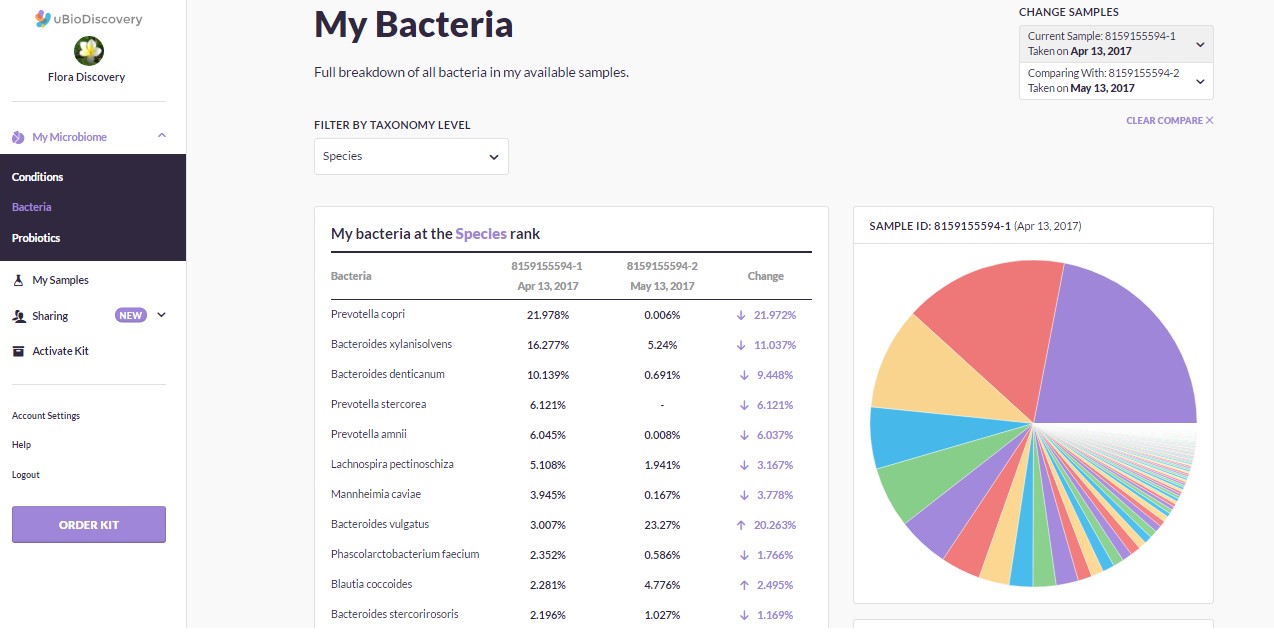
Superbiome results dashboard
How do we make sense of this information?
It is extremely difficult to fully understand the complex interactions between our own biochemical systems and those of the diverse microbial community living inside us. There are thousands of different species of bacteria which live in our gut, and the proportions of each are not the same in all individuals (even in those who are healthy). We don’t know what roles each of them play, but generally they act to break down food we eat, producing a chemical soup comprised of nutrients, waste products, enzymes, signalling molecules and other biologically active compounds that are implicated in our overall health.
These organisms should be in balance with each other. If an offending organism takes hold in our gut for some reason, beneficial organisms may struggle to compete, leading to possible acute or chronic illness when the aforementioned chemical soup becomes unbalanced. The exact factors that define a person’s microbiome are not known, but once it is established in an individual it is resistant to change. There is evidence that a combination of environmental and genetic factors play a role (eg. Microbial exposure at an early age, foods eaten, infection, antibiotic use).
What can we do to change our microbiome?
Current research is trying to uncover what this microbial community looks like in a healthy person versus a person with a disease. Researchers have discovered there may be a limited number of well-defined and balanced microbial states (called enterotypes). Each enterotype may respond in a specific way to diet or drug intake. Large-scale projects such as the Human Microbiome Project seek to generate the data necessary to uncover how to define these enterotypes, and create new methods of treatment for various gut microbiome-related diseases by restoring the ‘ideal’ enterotype on an individual basis.
If we can definitively link specific enterotypes to specific disease states, the next step is determining how we can adjust the microbial community in our gut to see if we can treat disease. Some ways this could be done include probiotics (ingesting beneficial microorganisms), prebiotics (ingesting food that beneficial microorganisms need to thrive), antibiotics (reducing the number of ‘bad’ microorganisms) and fecal transplants (replacing the microbial community entirely with another one).
Probiotic supplementation may act to stimulate the immune system through a complex mechanism. The benefits of this stimulation may include better infection control, improved lactose metabolism, anti-cancer mechanisms, reduction in cholesterol, and improvement in inflammatory bowel disease.
Fecal transplantation sounds like an extreme option (yes, it is what you think it is), but evidence seems to indicate positive results in patients with recurrent C. difficile infection, and other conditions like Crohn’s, metabolic syndrome and inflammatory bowel disease. However, it does seem that the transplanted microbiome may not permanently establish itself in the recipient in the long term. More study is required. In an extreme case, a desperate and curious scientist named Josiah Zayner sought to treat himself with a home-made fecal transplant and even made a short documentary about it. Don’t try this at home!
What diseases are related to the microbiome?
Cancer
There is evidence that high protein diets could increase the chance of colorectal cancer due to factors such as inflammation and carcinogenic compounds released by gut bacteria when they break down proteins. There may be other chemicals implicated in this disease as well. An imbalance causing an increased number of organisms that produce these compounds would raise the risk.
Obesity and Diabetes
Part of the job of the gut bacteria is to help break down our food in ways that allow our body to absorb the nutrients. Some bacteria are more efficient at this than others. If a person’s microbiome consists of a higher proportion of bacteria that are efficient at breaking down food, more energy is extracted from the food we eat and is stored as fat. In animal studies, when gut bacteria from obese mice are transplanted into the gut of regular mice, the regular mice become obese since they were able to extract more energy from their food.
Chemical compounds like bacterial lipopolysaccharide are created in the gut by microbes. Too much of this over a long period of time can lead to low level inflammation which is thought to be related to diseases like type 2 diabetes as well as obesity
Depression, anxiety, schizophrenia, bipolar disorder
Central nervous system conditions can be tied to disorders of the gut, as neurotransmitters and related molecules exist both in the gut and brain and are therefore linked (the gut-brain axis). Some refer to the gut as the ‘second brain’ for this reason. Some bacteria may produce or affect the production of neurotransmitters and other neuro-active chemicals in the gut which may affect psychological health.
Autism
Similar to the above conditions, there has been research implicating gut microbiome health with the development of autism spectrum disorders.
Irritable Bowel Syndrome (IBS)
IBS is a collective term for a range of gastrointestinal symptoms that can’t be attributed to a specific disease. Many people suffer from abdominal pain, gas and bloating, constipation, diarrhea and nausea either chronically or when they consume certain foods. Some of these symptoms may be explained by an imbalance in a person’s gut bacteria. There is evidence that certain probiotics help alleviate these symptoms.
Promising, but early science
When a new, exciting scientific study comes out, the internet tends to over-react with clickbait headlines, promises of miracle cures endorsed by popular celebrities and general misinterpretation of the science. Let’s not do that here. The connections and mechanisms between gut bacteria communities and disease is still being worked out. Realizing the potential, giants like IBM are partnering up with huge research institutions to try and figure this out.
The ability to specifically alter these bacterial communities to treat disease is still at a very early stage. Microbiome researchers and companies like uBioDiscovery, uBiome, IBM and many more are working to characterize the relationships between gut bacteria and these health conditions with the goal of developing novel treatment and prevention strategies. Until the details of these new treatments are figured out, taking probiotics and eating a varied, healthy and high fibre diet are thought to positively regulate the gut microbiome. For any specific condition or medical concern, consulting with a doctor or GI specialist is still the best course of action.




